Study time: 7 minutes Publication date: 06/07/1401
Characteristics of maltodextrin :
Native starch has a limited industrial application due to its low functional properties, and to increase their application, physical, chemical or enzymatic changes must be made in the starch molecule and increase the range of products with special characteristics, which can be used wide range of applications in various fields of food and pharmaceutical industry (Rocha et al., 2005). An easy and fast way to obtain carbohydrates with specific functional properties is through starch hydrolysis.
Dextrose equivalent (DE) is degree of hydrolysis of the starch molecule, defined as direct reducing sugar (ARD) content, expressed as a percentage of glucose on a dry matter basis. Depending on the degree of hydrolysis of the starch molecule, a wide range of products is obtained, which are classified into maltodextrins and syrups according to the (DE) content; Maltodextrins have DE < 20, while syrups have DE ≥ 20 (McPherson and Seib, 1997).
Maltodextrin [(C6 H10O5) nH2O] is recognized by the Food and Drug Administration (FDA) as a mixture of nutritious, non-sweet carbohydrates with varying degrees of polymerization, consisting of D-glucose units linked by α (1,4) and α (1, 6) glycosidic linkages, defined and have DE < 20. Maltodextrins are available as white powders or concentrated solutions and are generally classified as safe food additives (GRAS) (Marchal et al., 1999; Storz and Steffens, 2004; Storz and Steffens, 2004; Dokic-Baucal et al., 2004). ; Gibiński 2008; Muntean et al. 2010).
The average molecular weight and degree of hydrolysis of maltodextrins changes with dextrose equivalent (DE). In other words, the DE value of maltodextrin generally varies between 0 and 20. (Archilla, 1999) As a result, when the DE value of maltodextrin increases, the molecular weight decreases. Maltodextrins with different DE values show different physicochemical properties. More moisture absorption is seen in maltodextrin with higher DE and lower molecular weight (Archilla, 1999). In addition, the results of other studies show that maltodextrins with higher DE content have higher solubility and sweetness (Chug et al., 2013). Also, with an increase in the amount of DE, moisture absorption, solubility, osmolality (concentration of dissolved solids in a solution) and their effectiveness in reducing the freezing point increase, while viscosity, adhesion and preventing the formation of coarse crystals increase with a decrease in the amount of DE (Dokic- Baucal et al., 2004; Y.-J. Wang & Wang, 2000) even maltodextrins with the same DE value may show very different physicochemical properties due to differences in maltodextrin production(hydrolysis), starch source, and amylose/amylopectin ratio (Dokic-Baucal et. al., 2004).
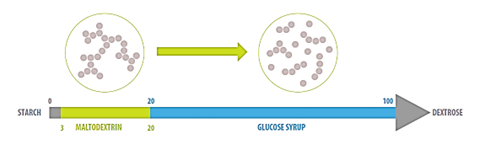
It is assumed that the functional characteristics of maltodextrins and the degree of DE are related, and it is practically used as a guide to determine their applications. For example, maltodextrins with DE10 are commonly used for flavor carriers, flavoring encapsulation, instant sauces, and diet and light products. Maltodextrins with DE 15 are used for isotonic drinks, dry soups powder, and maltodextrins with DE 20 are used for chocolate powder, dairy desserts, beverage powder, and industrial bakery premixes.
The main commercial sources of starch for the industrial production of maltodextrin are: corn, potato and rice, but they can also be prepared from a variety of starch materials such as tapioca, wheat, sorghum, etc., depending on the availability and price of the raw materials produced in every country (Jimenez et al., 2007; Antonio et al., 2009; Jing et al., 2011)
Compared to raw starch, maltodextrin is more soluble in water and is cheaper than other main edible hydrocolloids, and its solutions are colorless, have a mild taste and soft texture in the mouth (Dokic-Baucal et al. 2004).
Production of Maltodextrin :
Maltodextrins are obtained industrially by controlled hydrolysis of starch, using acids, enzymes or a combination of both (Lumdub wong and Seib, 2001). Today, the acid hydrolysis method is less used in the industry. Acidic methods are mostly used to prepare glucose syrup. It is difficult to produce syrup or powder less than 30% DE through the acid hydrolysis process due to the formation of non-degradable and stable starch in the crystalline state. In the continuous processes of producing hydrolyzed starch products, enzymatic hydrolysis methods or a mixture of acid and enzyme have replaced acid hydrolysis. The use of enzyme in the hydrolysis process is more widespread due to several advantages compared to the use of acid solution. Enzyme method has outstanding advantages compared to acid process. Enzyme hydrolysis equipment is easier than production using acid, which requires acid-resistant equipment. No need to remove salts formed during acid neutralization, enzyme activity in a wider pH range and at lower temperatures than
acid hydrolysis (with obvious energy savings), higher efficiency, easier process control, and also during the hydrolysis process of inferior materials Less produced (Haki and Rakshit 2003).
Carbohydrate profile of maltodextrins obtained from hydrolysis, i.e., its average degree of polymerization (GPP), linearity and degree of branching of their constituent carbohydrates, influenced by source and concentration of starting starch, conditions (temperature and time) as well as type and concentration of enzyme The method used in this process is hydrolysis. This means that there may be maltodextrins with the same DE, but different molecular composition, linearity and branching of carbohydrate incorporation, providing different physicochemical properties and performance for each of them. (Chronakis, 1998; Marchal et al., 1999). Based on differences in the chemical composition and structure of the starting starch, the enzymatic hydrolysis time required to obtain the desired maltodextrin with DE will be different for each type of starting starch. The dextrose equivalent (glucose equivalent) of maltodextrin is related to the ratio of amylose and amylopectin content in the starch used for its production. A higher amylopectin content is associated with a higher dextrose equivalent of maltodextrin. The ratio between linear amylose chain molecules and branched amylopectin chain varies according to the nature of starch. Most starches contain between 15 and 35% amylose. The enzymatic starch hydrolysis process is shown in Figure 2.
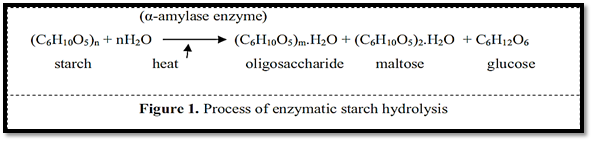
Applications of Maltodextrin :
Maltodextrin is used for multiple purposes in food products: providing nutritional value, improving texture, binding to flavor and fat components, protecting against oxygen, providing surface shinning, aiding dispersibility and solubility, increasing soluble solids, inhibiting crystallization, controlling Freezing point, filling, bulking, creating consistency and texture, controlling sweetness and moisture, preventing crystallization and non-enzymatic browning, regulating osmolality, excellent fat substitutes, film forming without coating effect on flavors (Chronakis, 1998; Herrera et al., 2000; Wang and Wang, 2000) Maltodextrin can also be used as an anti-caking agent and suitable filler in the production of spray-dried foods (Chronakis 1998; Setser and Racette 1992; Alexander 1992).
Since maltodextrins are very hydrophilic, they can form gels. Therefore, they are more preferable for use in the food industry as texture modifiers, thickeners and fat replacer.
When sufficient water is present on the surface of the material, maltodextrin exhibits fat-like properties and forms a gel-like matrix that results in the product’s lubricity and flow properties. This gel-like matrix results from interactions between amylose segments characterized as helical regions with linear and branched chains of amylopectin molecules (Chronakis, 1998). Because maltodextrins are very small in diameter (3-5 µm), they resemble fat crystals, leading to maltodextrin-like fat behavior, and a favorable mouth feel in foods. This feeling probably originates from the three-dimensional network of submicrons that exist in the structure of water layers. Therefore, maltodextrin can be used in emulsions as a texture modifier, bulking agent, and especially in food emulsions to some extent as a fat replacer. For this reason, it is preferred in low-fat food systems, especially for the preparation of spreads, margarine, salad dressings, baked goods, fillings, sauces, and processed meats (Chronakis, 1998).
In particular, maltodextrins are not surfactants and thus their main stabilizing action in oil-in-water emulsions is believed to be through viscosity modification or gelation of the aqueous continuous phase surrounding the oil droplets (Dickinson, 2003). Therefore, emulsions containing maltodextrin as a stabilizer need an additional emulsifying agent to produce a stable emulsion (Hogan, S.A., et al., 2001). In addition, molecular characteristics of maltodextrin, such as concentration and chain length, affect the overall rheology and stability of oil-in-water emulsions.
Maltodextrin is sometimes taken as a supplement by bodybuilders and other athletes in powder or gel packs. Maltodextrins considered to be a good source of energy for infants and athletes because the body digests Maltodextrins as a simple carbohydrate, so they are easily absorbed in the small intestine and are quickly available for use by the body. Due to this characteristic of maltodextrin, it is used in sports drinks and special sachets to get quick energy for endurance athletes and to increase the calorie content of baby food. They can also help balance intestinal osmolality in children which may be altered by intestinal disorders in infants (Gregorio et al., 2010). And athletes benefit from a variety of dextrose levels thanks to a variety of Maltodextrins. In addition, Moreover, maltodextrins are suitable for infant nutrition as they are easy to use. Their solubility ensures a lump-free formula for bottle-feeding and gives milk a perfect consistency.
Maltodextrin-based products, especially soy products, soy milk powder for babies, are usually used for children with lactose intolerance or allergy (Consenso Brasileiro, 2007; Raju AS, et al., 2012). These formulas are used as a substitute for cow’s milk. Regulations governing infant nutrition include maltodextrins in the list of authorized carbohydrates for use in food intended for infants (EU Regulation No. 609/2013 and Delegated Regulation (EU) No. 2016/127).
Also, according to the rules of the Celiac Disease Foundation, maltodextrin is considered gluten-free, although wheat-derived maltodextrin can contain small amounts of gluten, but corn-derived maltodextrin is gluten-free.
With the advancement of science and technology, knowledge about the functional possibilities of maltodextrin in food and beverage products has improved significantly over the 20 past years. Due to specific technical/functional properties and easy application, maltodextrin can replace sucrose (O’Brien-Nabors, 2011) or fat (Alexander, 1995; Hadnađev et al., 2011) and in ice cream and dried food formulations, Sweets, cereals, snacks and instant drinks are used. (Takeiti et al., 2010)
Nutrition and health experts recommend that the daily consumption of fat should not exceed 30% of the total calories in the diet, because failure to comply with this issue leads to endangering people’s health and the spread of diabetes and cardiovascular diseases in the society, for this reason in recent years the demand for dietary and low-fat food products as well as with reduced sugar has caused researchers to pay attention to low-calorie products (reduced or replaced with fat or sugar). Maltodextrin has been one of the most popular carbohydrate-based fat substitutes for the past 30 years. If maltodextrin is used in its pure form, it will produce 4 KCal⁄g of energy, if it is usually mixed with water at a ratio of one to four, as a result, compared to 9 KCal⁄g of fat, it will produce about 1 KCal⁄g less energy. Therefore, in addition to creating a greasy and favorable mouth feel, it will reduce the energy of the product. The use of Maltodextrin in food production can reduce calories by more than 70% (Marchal, L. M, et al., 1999. Replacing Fat with maltodextrin significantly reduces the energy content of the food (16 kJ vs. 38 kJ). Maltodextrin is used in “diet” peanut butter to reduce the fat content, but maintain its texture.
Research shows that one of the advantages of Maltodextrin is to prevent the release of volatile compounds, which makes it a suitable alternative to fat for use in low-calorie meat products. Also, Maltodextrin is widely used in dairy products such as ultra-filtration cheeses, types of yogurts, confectionary cream, ice cream, etc. as a fat substitute and texture improver.
The low glass transition temperature of the main compounds of low molecular weight sugars and organic acids, their hygroscopicity, low melting point and solubility in water make drying the extract and fruit juice difficult and lead to the production of sticky deposits on the wall of spray drier (Ferrari et al., 2012). The use of auxiliary materials with high molecular weight (carrier) can increase the glass transition temperature and reduce the amount of adhesion to the wall. Soy protein isolate, sodium-caseinate and vegetable proteins, etc. have been used as carriers in the food industry (Truong V, 2005), but in general, Maltodextrin with high solubility, low viscosity, low price, neutral taste and aroma., is a suitable wall material for protecting sensitive compounds to oxidation, by facilitating the spray drying process, and is the most used among other carriers. The lower the dextrose equivalent (DE), the higher the glass transition temperature and therefore the lower the adhesion of the final powder. In addition, a number of researchers have mentioned the increase in the density of the powder produced by the spraying method with the help of high dextrose equivalent maltodextrin (DE=18-20). The increase in density reduces the cost of transportation and increases the flowability when preparing a reconstituted drink (Shishir MRI and Chen W, 2017).
Some of the other applications of maltodextrin are in fillings and coatings, dairy products (including frozen desserts), meat products, bakery (sweets, snacks and cakes), confectionery, microencapsulation of color and flavor, etc. Maltodextrin can replace part of the oil as a coating in low-calorie salad oils.
Maltodextrin can be added in powder form to liquid ingredients in dough production. Maltodextrin mixes easily with natural oils and fats to form stable emulsions under refrigerated conditions. For this purpose, maltodextrin with low DE is more suitable. In frozen desserts, maltodextrin combined with cellulose gums prevents the formation of large crystals during freezing and can control crystallization and lower the melting point. Maltodextrin can be a useful barrier to reduce the non-enzymatic browning reaction (Millard) and be used in micro-
encapsulation of food components such as fats, oils, vitamins, minerals and color compounds. This application of maltodextrin is due to the characteristic of water absorption and gel formation. Microencapsulation is a technology in which fine particles and microencapsulated droplets (core) are surrounded and covered by a film made of coating material (wall), which protects the core, and in this way, microcapsule particles are created. The contents are left under favorable conditions so that useful properties can be obtained from it. The composition of the coating is a determining factor of the properties of the produced capsule. The compounds used in the micro coating process are in a range of natural or synthetic polymers. Coating materials should be selected in such a way that they produce an emulsion or a suitable dispersed solution with the active ingredients and should not react or decompose with the active ingredients during the process or storage. In addition, they should match the solubility properties of the capsule and the release properties of the active ingredients. Carbohydrates such as starch, maltodextrin, etc. are the best choice for micro coating applications due to their favorable physicochemical properties such as solubility and melting, different sizes and low price.
If maltodextrin is mixed with oils, due to the low viscosity of its solution, it does not increase the emulsification of the oil like other substances. Molecules with high DE protect the oil against oxidation. It is speculated that the capsule composed of maltodextrin, lecithin of egg yolk, gelatin and caseinate shows optimum protection against oxidation. The effect of maltodextrin is very important in protecting the taste against oxidation.
In the pharmaceutical industry, similar to the food industry, maltodextrin is used as a filler. They are usually present in capsules because they neither add nor decrease the content of the drug, while they stabilize it. Maltodextrin is used as a diluent in tablets and coating materials in microencapsulation of various sensitive substances such as vitamins and has other uses in the pharmaceutical industry.
Maltodextrin is used in cosmetics and skin and hair care products as an emulsifier, moisturizer and hydrating agent. It is used in shampoos and conditioners and in toothpaste as a texture improver. The use of maltodextrin in shampoos goes beyond being only a stabilizer. It can enhance the antiaging benefits of alpha hydroxy acids, or AHAs, commonly used in antiaging products. Maltodextrin also plays a role in improving the texture of products. Since maltodextrin is made of simple sugar units that are soluble in water and has the ability to create a gel-like texture in formulations, the presence of maltodextrin in shampoo makes it appear a lighter product and spread evenly on the hair. Also, it acts as a binding agent, helping to ensure that the formulation remains uniform throughout use and storage and even help the texture.
Recent research has shown that maltodextrin may have anti-aging properties. In 2002, a patent filed by a company called Unilever presented research on the use of maltodextrin in combination with hydroxyl acids. Hydroxyl acids such as alpha hydroxyl acids and beta hydroxyl acids are commonly used in skin and hair care products due to their ability to improve the appearance of skin and hair which damaged by light or natural aging processes. It helps reduce visible
pigmentation caused by hormones, genetics, sun and diet. An important issue with hydroxyl acids is that they can cause irritation such as redness and burning. Researchers found that although maltodextrin itself is not an ant aging compound, it can enhance the anti-aging activity of alpha hydroxy acids and reduce hair and skin irritation.
As a binder, maltodextrin also plays a role in binding other materials together and preventing them from separating. For example, binders are often used in compressed powders to hold them in the container.
It is used in the paper industry as an adhesive to improve the appearance and in the cement industry as an emulsifier.
Conclusion :
Considering the many properties and characteristics of maltodextrin and the approaches of industry and technology in the field of food, pharmaceuticals and cosmetics… many applications for maltodextrin can be imagined. Especially in the food industry, due to the change and improve of people’s culture regarding the use of substances with reduced sugar and fat, as well as the desire of food producers, according to the market demand and the development of global health, to reduce sugar and fat in food products to reduce non-communicable diseases., it is possible to predict a wide perspective for the use of maltodextrin in the industry.
Prepared and written by: Mandana Alipour
Director of Research and Development of Golshad Grain Refinery
The use of the contents of this article is permitted by mentioning the source.